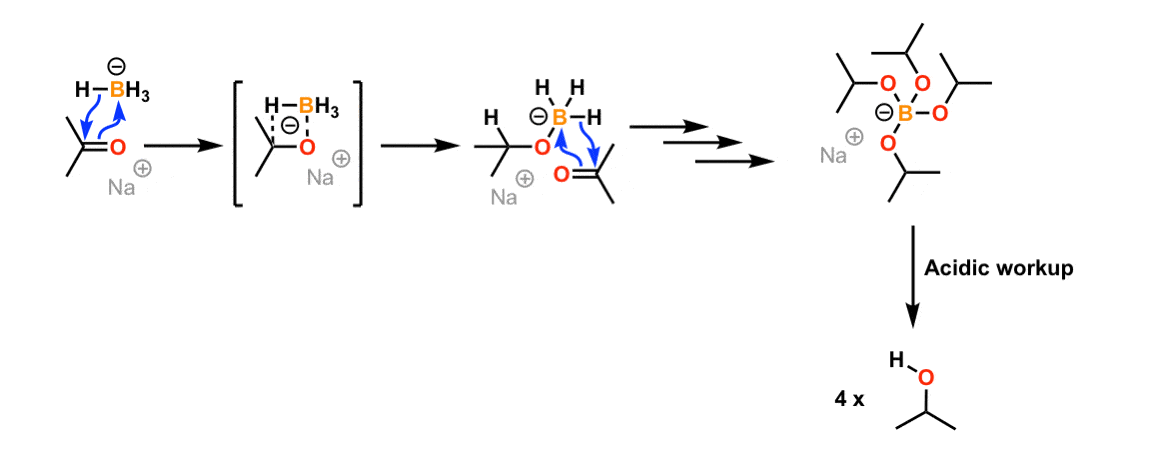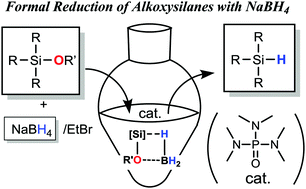
Synthesis of hydrosilanes via Lewis-base-catalysed reduction of alkoxy silanes with NaBH4 - Chemical Communications (RSC Publishing)

Reaction of InCl3 with Various Reducing Agents: InCl3–NaBH4-Mediated Reduction of Aromatic and Aliphatic Nitriles to Primary Amines | The Journal of Organic Chemistry

Sodium borohydride (NaBH4) reduction of m7G in RNA. Reduction of m7G... | Download Scientific Diagram
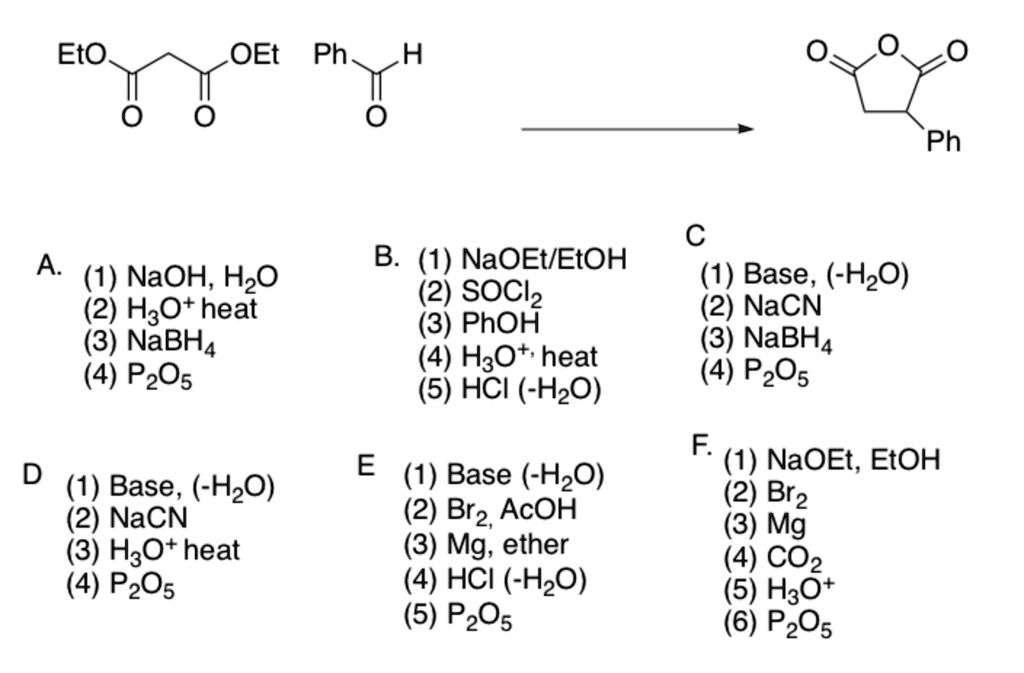
SOLVED: Eto OEt Ph H Ph C (1) Base, (-HzO) (2) NaCN (3) NaBH4 (4) PzO5 A NaOH, HzO (2) H3Ot heat (3) NaBH4 (4) PzOs B: (1) NaOEtIEtOH (2) SOClz 3)

organic chemistry - Is sodium borohydride really a better base than an alcohol? - Chemistry Stack Exchange


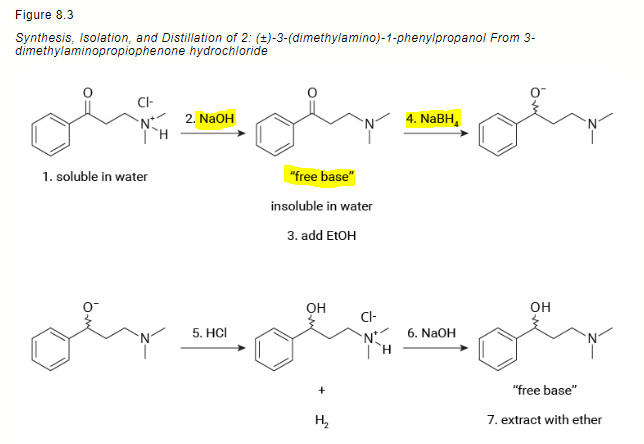
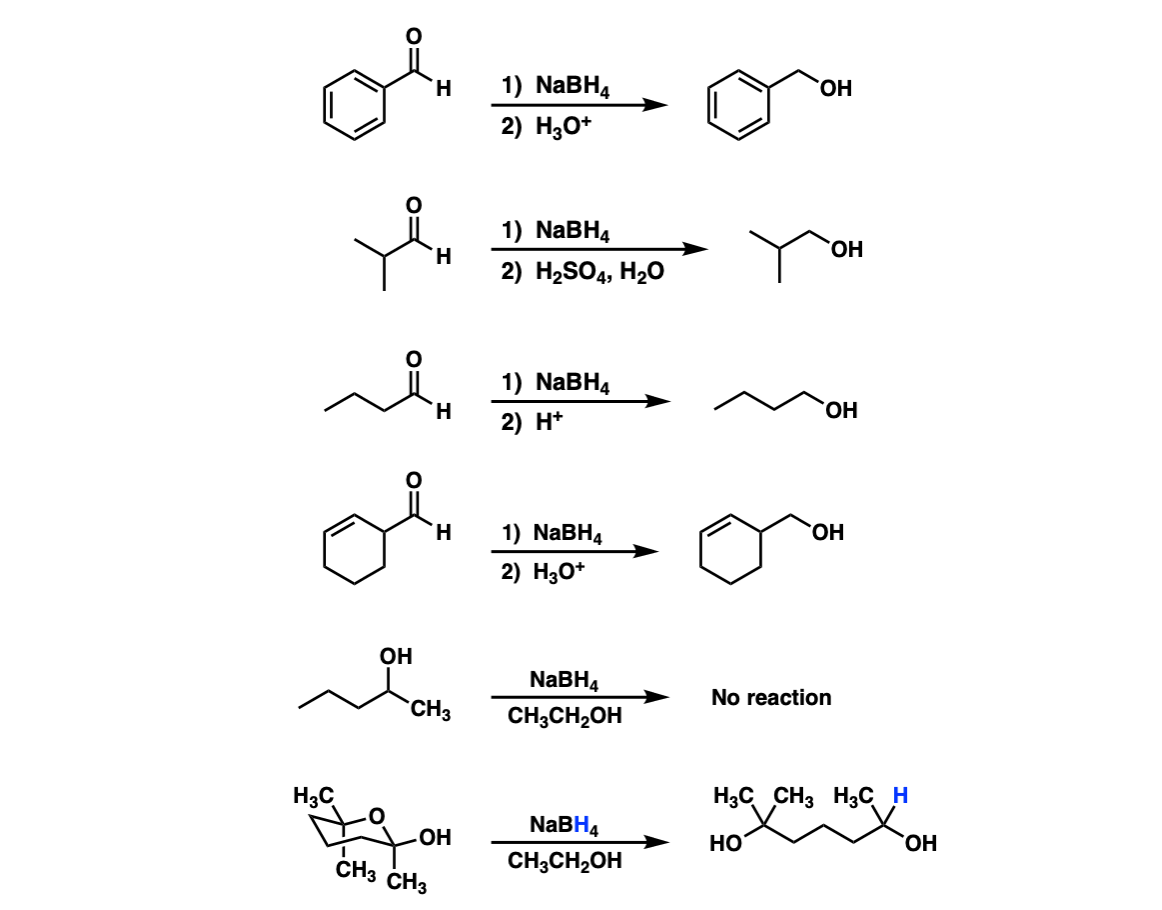

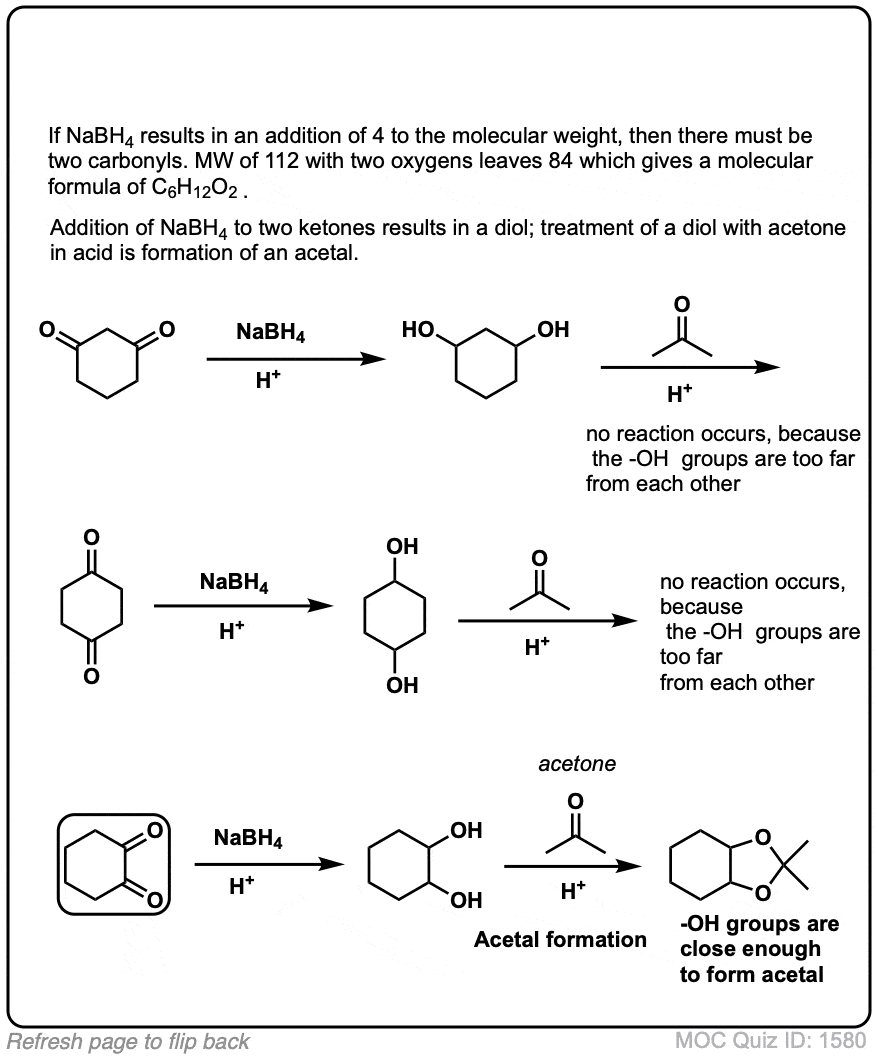
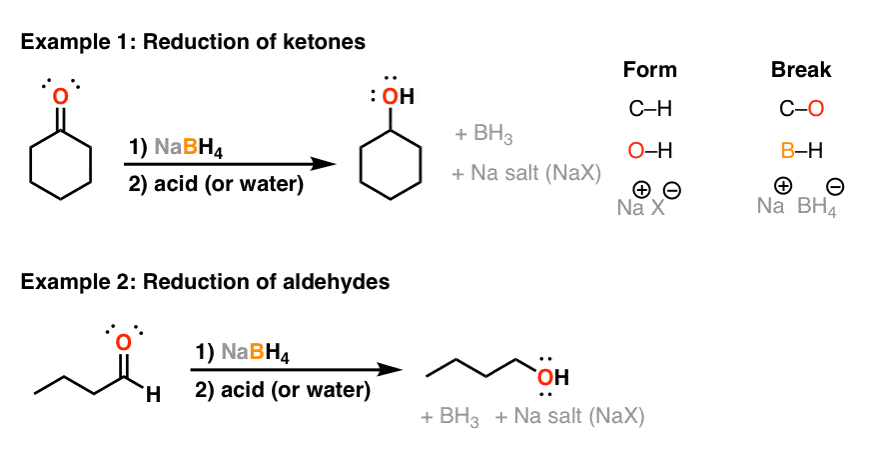

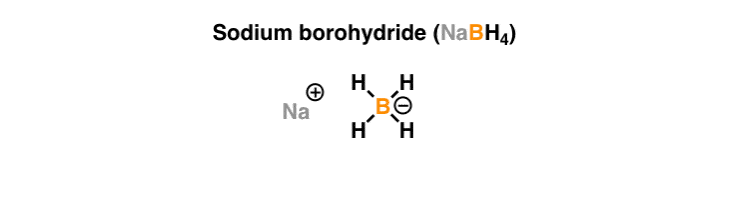

![Synthesis of compound 11. NaBH4: sodium borohydride.[³⁵] | Download Scientific Diagram Synthesis of compound 11. NaBH4: sodium borohydride.[³⁵] | Download Scientific Diagram](https://www.researchgate.net/publication/360210995/figure/fig4/AS:1149327540006912@1651032447935/Synthesis-of-compound-11-NaBH4-sodium-borohydride.png)