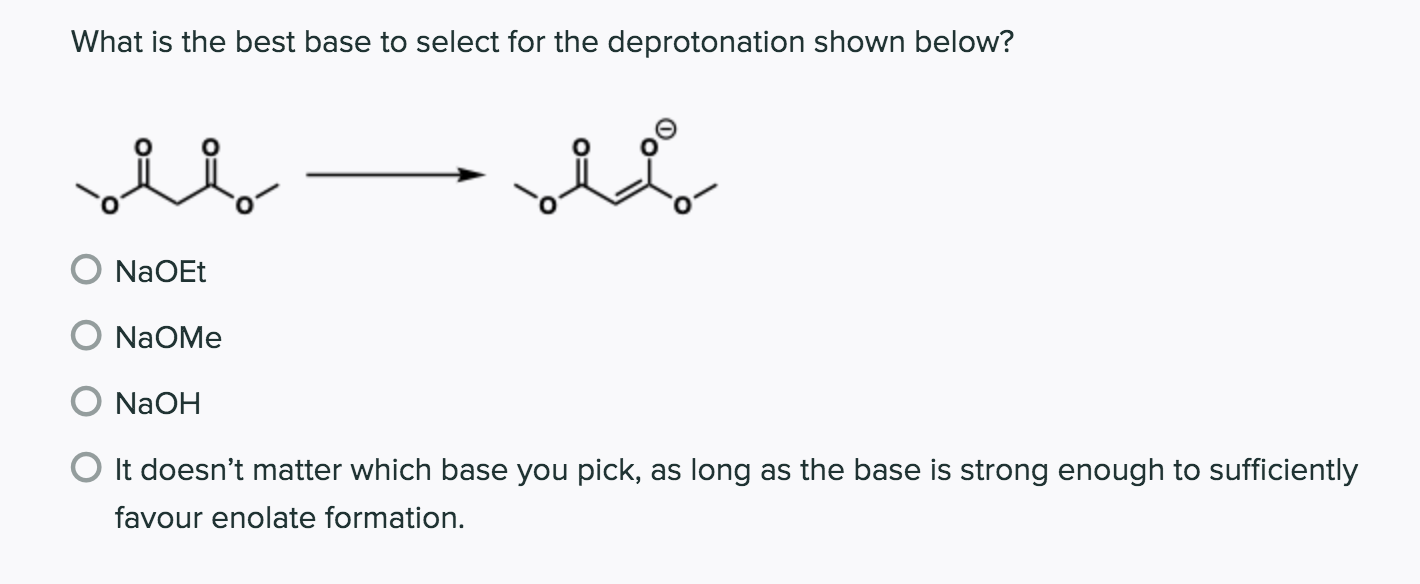
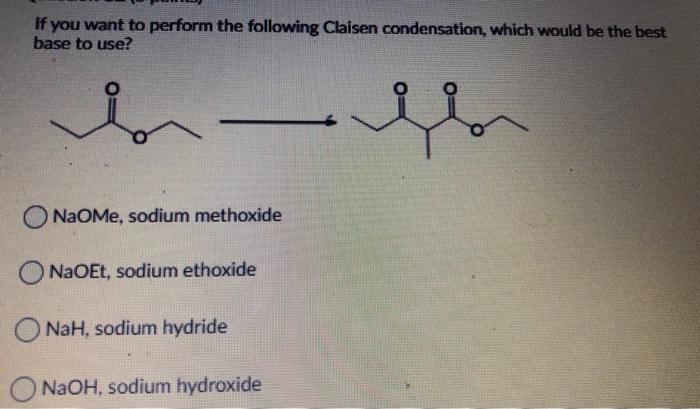
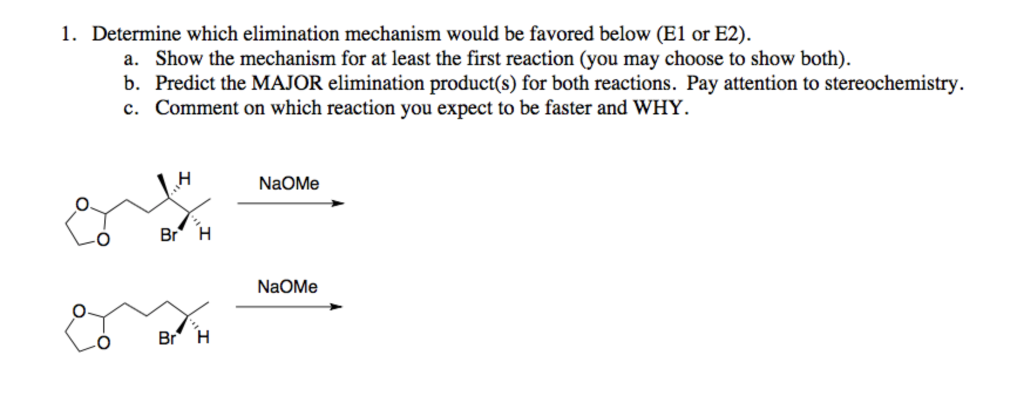
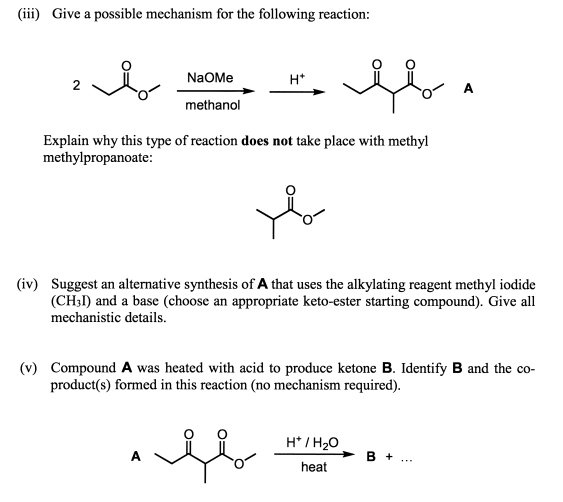
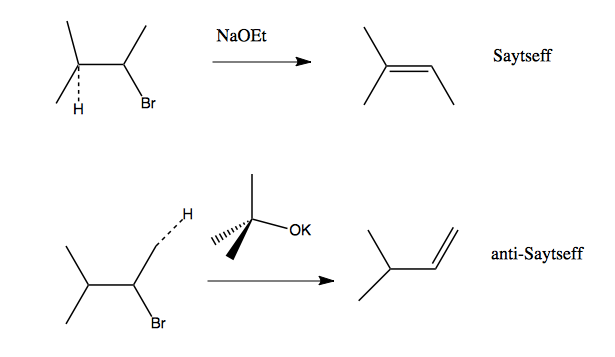
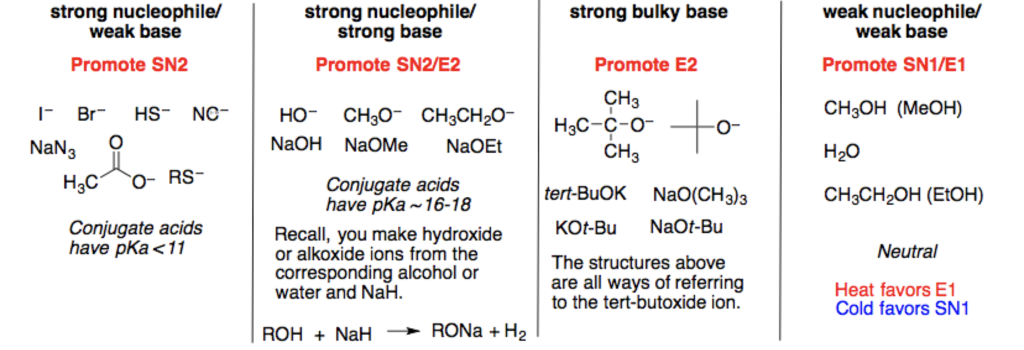
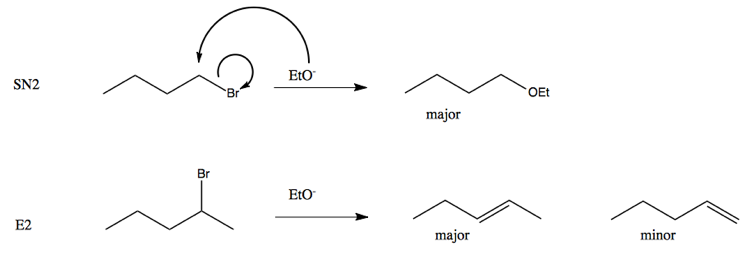
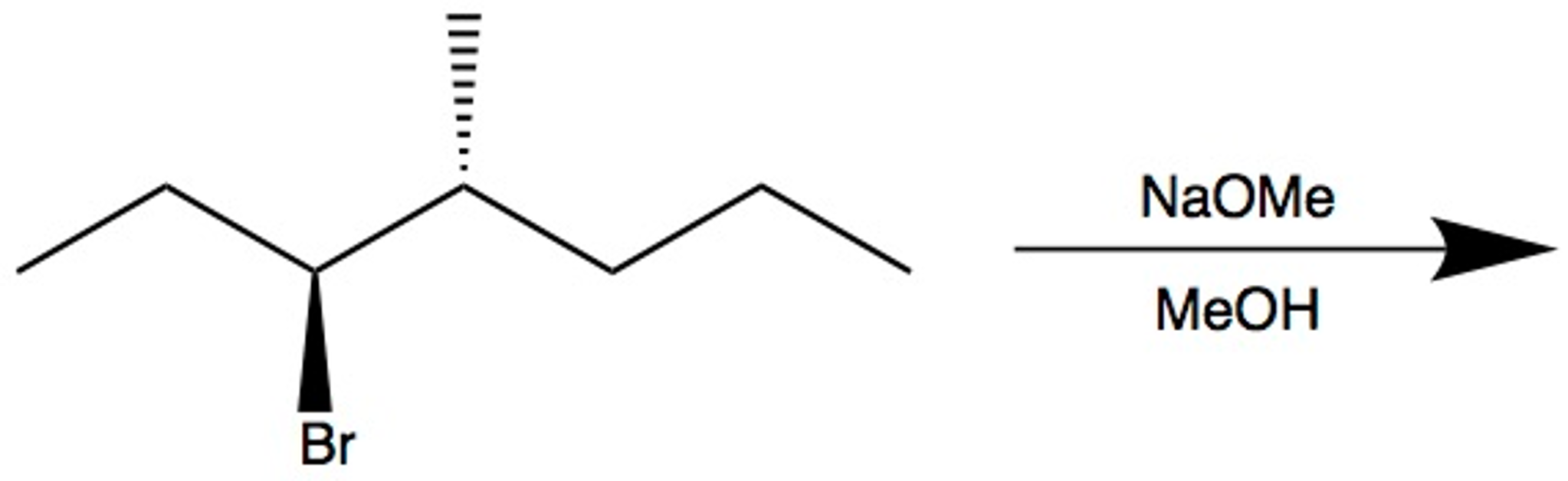
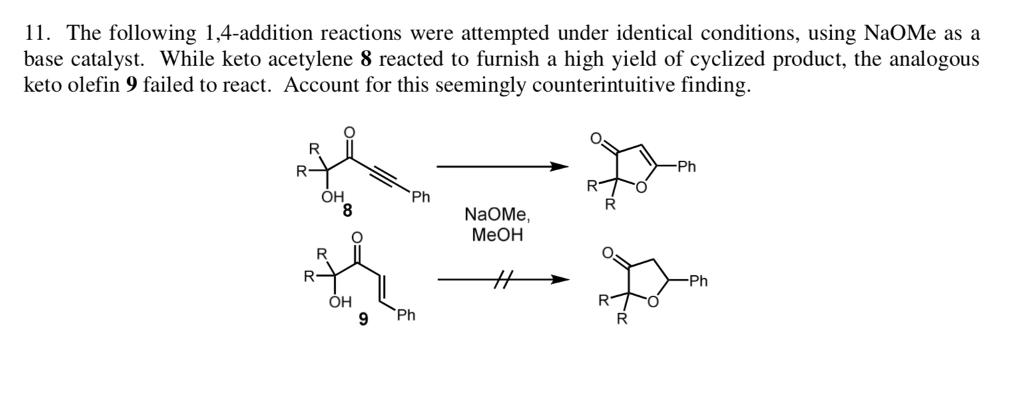
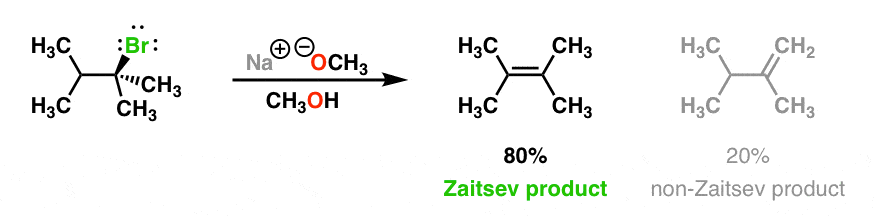
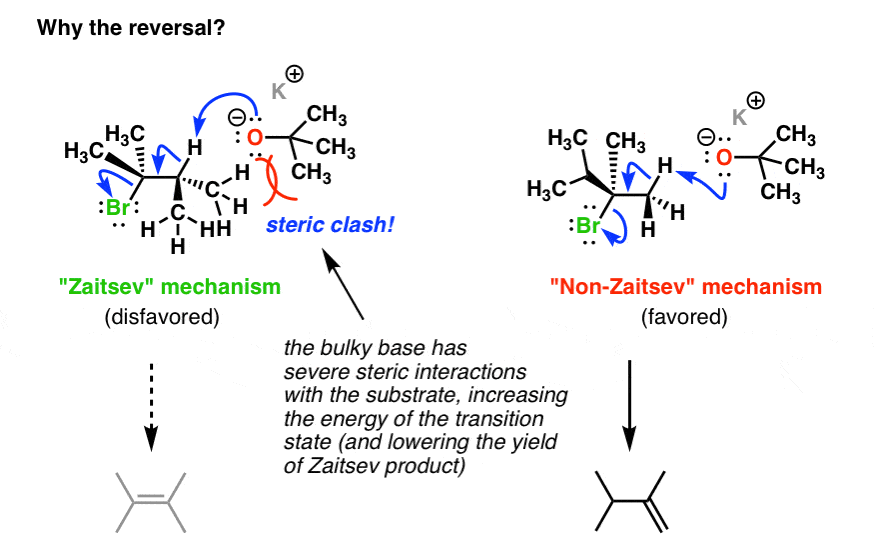
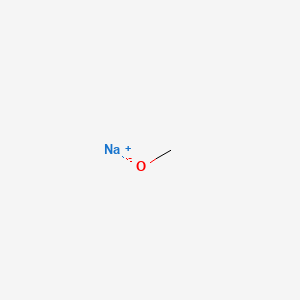
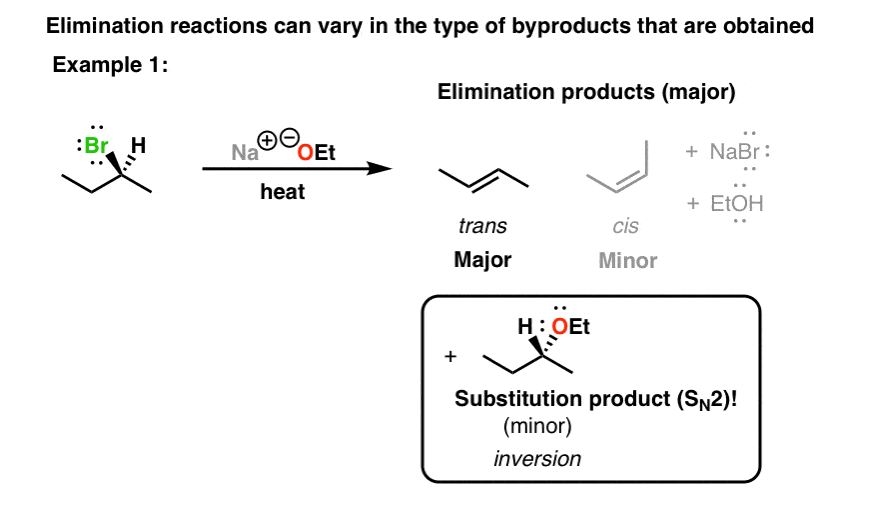
When we use a base in a reaction, why is it always preferred to use the conjugate as the solvent? For example, if NaOMe is my base, the solvent will be HOMe .

Supply structures for H through K. Given : " An Aldohexose "overset (MH2OH"/ base ")(to)H overset (Ae2O"/"NaOAC)(to)I overset (-HOAC)(to)J overset (NaOMe "/"MeOH)(to)K.

Scheme 2. Nucleophilic addition of NaOMe onto 1: acetal formation under... | Download Scientific Diagram

When we use a base in a reaction, why is it always preferred to use the conjugate as the solvent? For example, if NaOMe is my base, the solvent will be HOMe .

Here Meoh is used as a solvent and NaOMe is used as a nucleophile...then why is the product and elimination product rather than a substitution product?Thank u in advance community😁 : r/OrganicChemistry
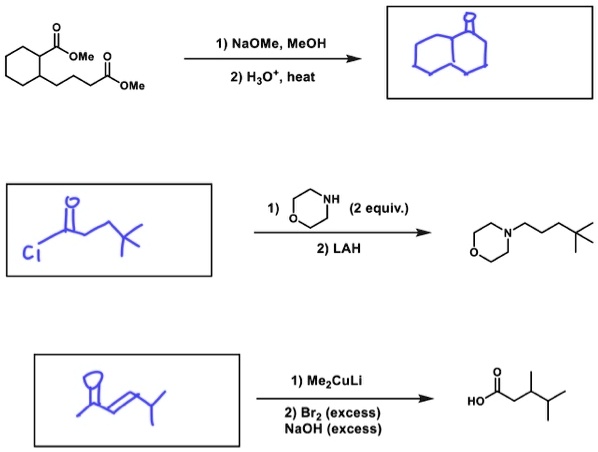
SOLVED: 1) NaOMe, MeOH OMe 2) H3O' , heat OMe (2 equiv:) 2) LAH MezCuLi Brz " (excess) NaOH (excess)

Scheme 2. Nucleophilic addition of NaOMe onto 1: acetal formation under... | Download Scientific Diagram