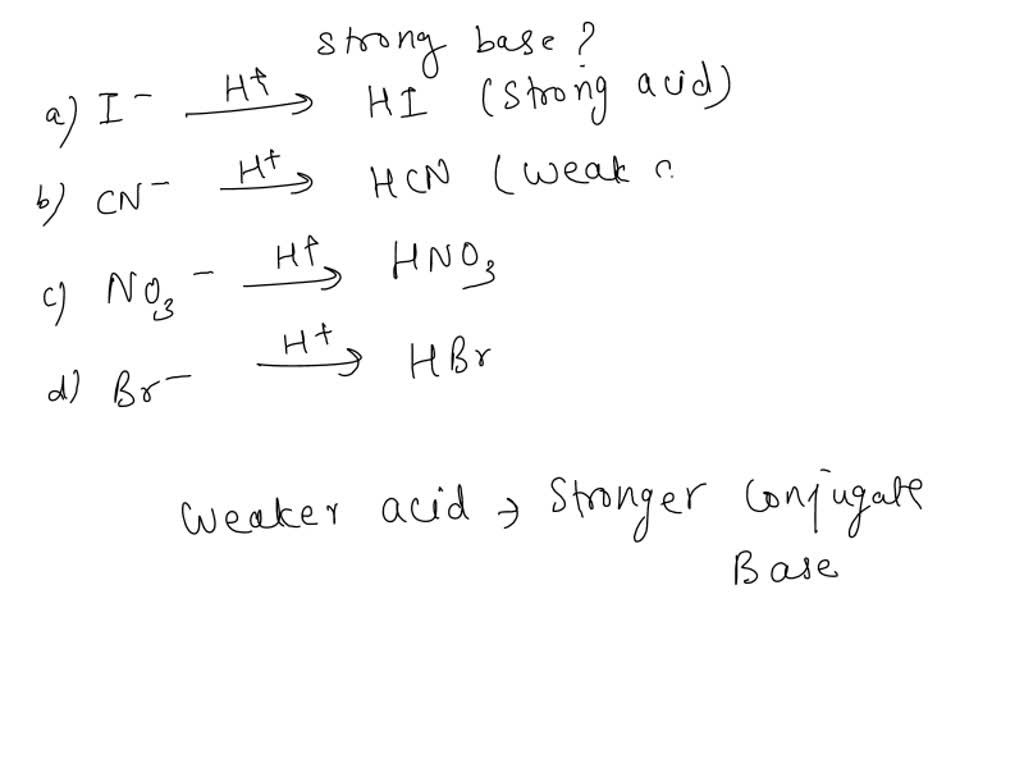
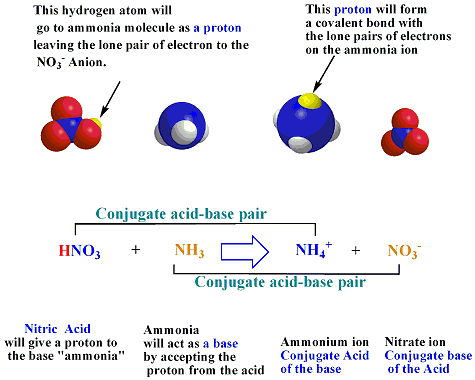
⚗️Identify the acid, base, conjugate acid, and conjugate base in the following reaction. HNO3 + NH3 + - Brainly.com

OneClass: 26 Which set is a conjugate acid/base pair? Marks: 1 Choose one answer a. HNO3 NO2 b. HNO2 ...
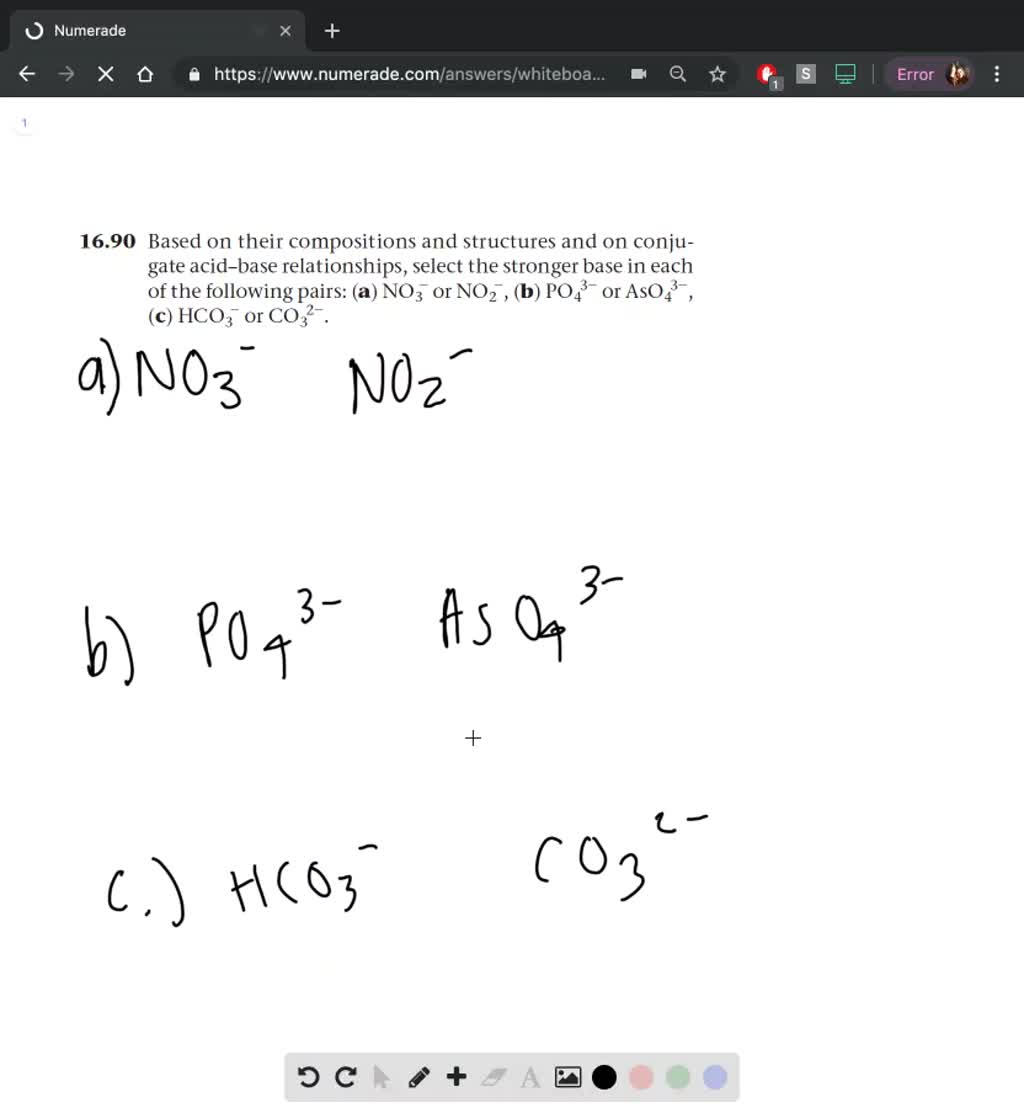
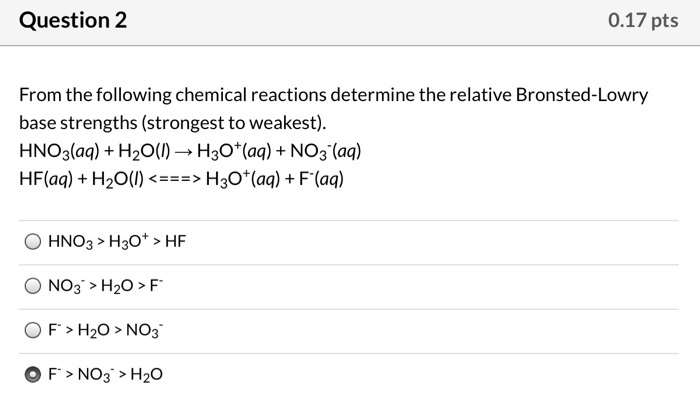
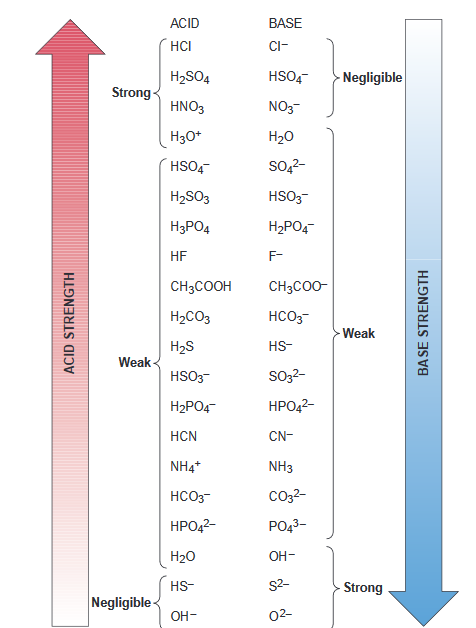
SOLVED:Based on their compositions and structures and on conjugate acid-base relationships, select the stronger base in each of the following pairs: (a) NO3^- or NO2^-,(𝐛) PO4^3- or AsO4^3- (𝐜) HCO3^- or CO3^2-.
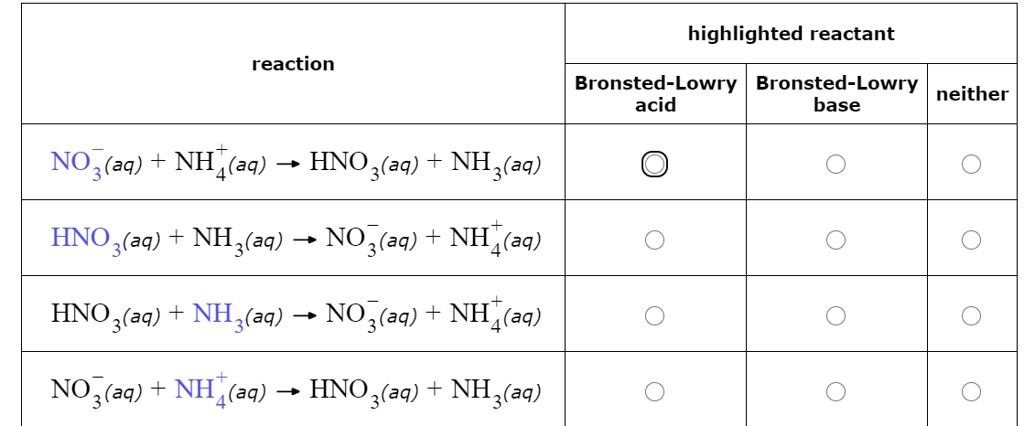
SOLVED: highlighted reactant reaction Bronsted-Lowry Bronsted-Lowry neither acid base NO3(aq) + NH4(aq) HNO 3(aq) NH,(aq) HNO 3(aq) + NH3(aq) 3 NO3(aq) NH A (aq) HNO 3(aq) + NH3(aq) 3 NO3(aq) NH A (

Base-Assisted Nitrate Mediation as the Mechanism of Electrochemical Benzyl Alcohol Oxidation | The Journal of Physical Chemistry C
Welcome to Chem Zipper.com......: How many nitrate groups are present in 1 molecule of basic beryllium nitrate?
Welcome to Chem Zipper.com......: How to basic beryllium nitrate is obtained from beryllium nitrates?

For the reaction below, identify the Bronsted-Lowry acid, the Bronsted-Lowry base, the conjugate acid, and the conjugate base. HNO3(aq) + H2O(l) arrow H3O+(aq) + NO3-(aq) | Homework.Study.com


![Mixing curve of low base flow [NO3 -] with shallow flow [NO3 -]... | Download Scientific Diagram Mixing curve of low base flow [NO3 -] with shallow flow [NO3 -]... | Download Scientific Diagram](https://www.researchgate.net/publication/346661459/figure/fig4/AS:965884747198464@1607296277040/Mixing-curve-of-low-base-flow-NO3-with-shallow-flow-NO3-superposed-onto-weekly.png)