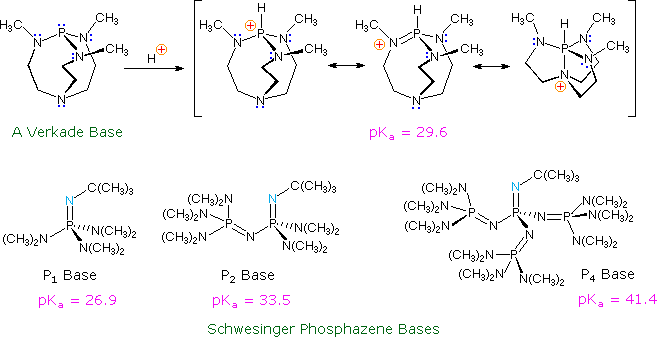
Verkade Base in FLP Chemistry–From Stoichiometric C–H Bond Cleavage to the Catalytic Dimerization of Alkynes | Organometallics

Interactions of Verkade's Superbase with Strong Lewis Acids: From Labile Mono- and Binuclear Lewis Acid–Base Complexes to Phosphenium Cations | Inorganic Chemistry

Interactions of Verkade's Superbase with Strong Lewis Acids: From Labile Mono- and Binuclear Lewis Acid–Base Complexes to Phosphenium Cations | Inorganic Chemistry

Interactions of Verkade's Superbase with Strong Lewis Acids: From Labile Mono- and Binuclear Lewis Acid–Base Complexes to Phosphenium Cations | Inorganic Chemistry
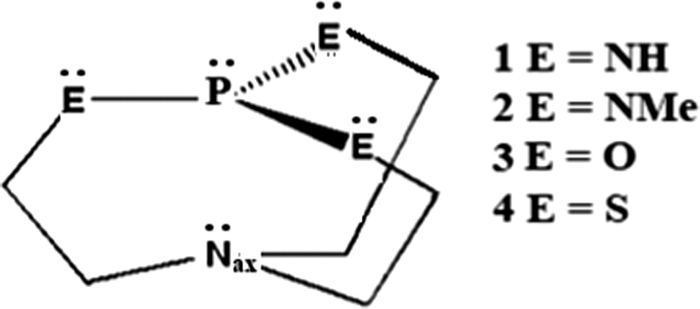
Protonation of Verkade bases: a theoretical study - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C8NJ04977G
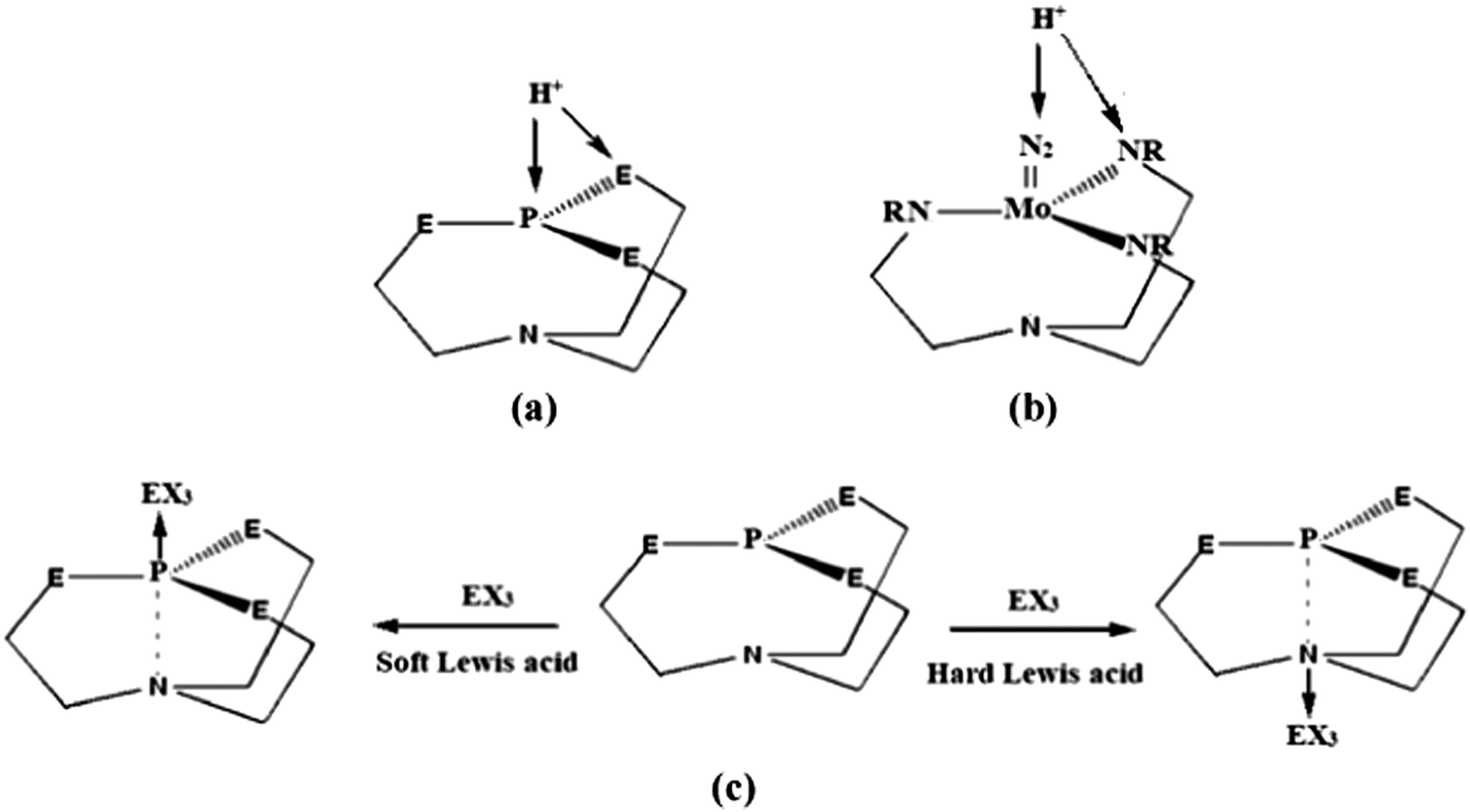
Protonation of Verkade bases: a theoretical study - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C8NJ04977G

Verkade Base in FLP Chemistry–From Stoichiometric C–H Bond Cleavage to the Catalytic Dimerization of Alkynes | Organometallics

Interactions of Verkade's Superbase with Strong Lewis Acids: From Labile Mono- and Binuclear Lewis Acid–Base Complexes to Phosphenium Cations | Inorganic Chemistry

Verkade Base in FLP Chemistry–From Stoichiometric C–H Bond Cleavage to the Catalytic Dimerization of Alkynes | Organometallics

Interactions of Verkade's Superbase with Strong Lewis Acids: From Labile Mono- and Binuclear Lewis Acid–Base Complexes to Phosphenium Cations | Inorganic Chemistry

Verkade Base in FLP Chemistry–From Stoichiometric C–H Bond Cleavage to the Catalytic Dimerization of Alkynes | Organometallics

Two Verkade's super bases (Vkd_Me and Vkd_iPr) and two guanidine bases... | Download Scientific Diagram
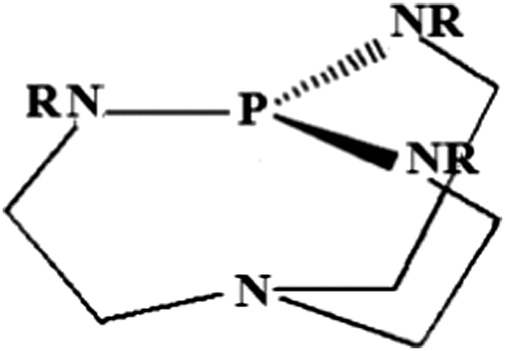
Protonation of Verkade bases: a theoretical study - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C8NJ04977G

Figure 1 from Very strong organosuperbases formed by combining imidazole and guanidine bases: synthesis, structure, and basicity. | Semantic Scholar

Lot - Kees Verkade, (b. 1941 Dutch), Two figures perfoming handstands, 1969, Patinated bronze on white marble base, Bronze: 11" H x 11" W x 8

Interactions of Verkade's Superbase with Strong Lewis Acids: From Labile Mono- and Binuclear Lewis Acid–Base Complexes to Phosphenium Cations | Inorganic Chemistry

Verkade Base in FLP Chemistry–From Stoichiometric C–H Bond Cleavage to the Catalytic Dimerization of Alkynes | Organometallics
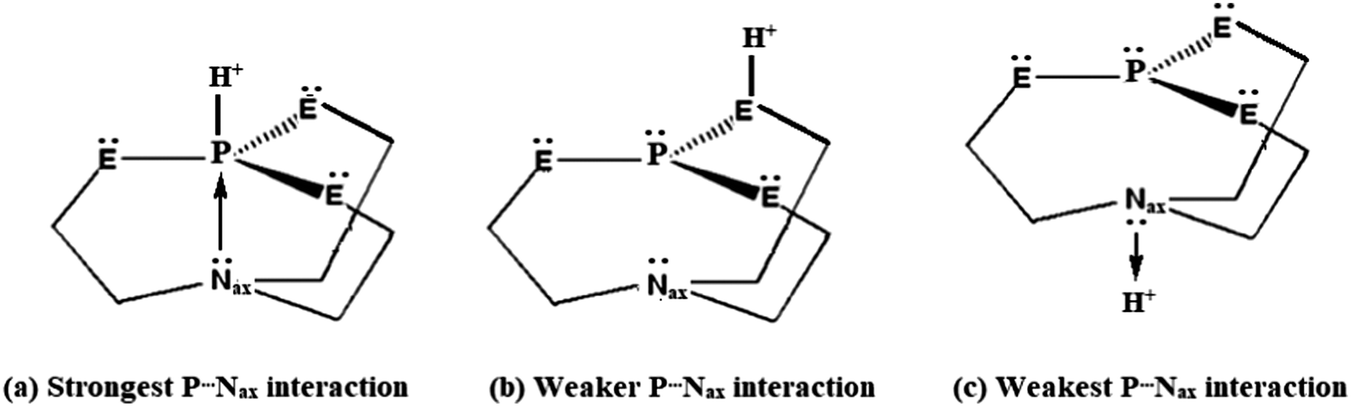
Protonation of Verkade bases: a theoretical study - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C8NJ04977G